Allelopathic effects of Juglans regia leaf extract on seed germination and seedling growth of wheat (Triticum aestivum) and rye (Secale cereale)
DOI:
https://doi.org/10.26832/24566632.2022.070102Keywords:
Agrochemicals Allelopathic, Concentration, Mortality, SeedlingAbstract
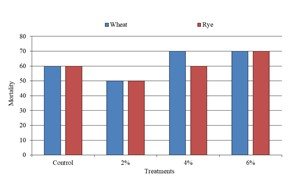
This investigation was conducted to study the allelopathic effects of Juglans regia (Walnut) leaf extract on seed germination and seedling growth of wheat (Triticum aestivum) and rye (Secale cereale). For this J. regia leaves extract was selected to analyze its allelopathic effects on seed germination and seedling growth of wheat and rye seeds. Applied seeds was treated with J. regia leaves extract using 2%, 4% and 6% concentrations. Different parameters i.e., seed germination, seedling growth, mortality percentage, fresh and dry weight of plumule and radicle was observed after the experiments. The higher seed germination percentage (50%) was recorded at 2% level, in both the applied seeds, which is followed by 4% in comparison to control. Almost higher concentration showed deleterious effects than the lower doses on germination percentage and seedling growth. The highest mortality percentage (70%) has been recorded at 4% level in wheat and 6% level in both wheat and rye seeds. Therefore, the results indicated that the growing weeds in high quantity between crops can affect the productivity rate of crops due to its allelopathic effects. The allelopathic compounds can be used as natural herbicides and other pesticides; they are less disruptive of the global ecosystem than are synthetic agrochemicals.
Downloads
References
Abu-Romman, S., Shatnawi, M., & Shibli, R. S. (2010). Allelopathic effects of spurge (Euphobia hieroslymitana) on wheat (Triticum durum). American-Eurasian Journal of Agricultural and Environmental Science, 7(3), 298-302.
Bhadoria, P. (2011). Allelopathy: A natural way towards weed management. American Journal of Experimental Agriculture, 1, 7–20.
Bhatt, B. P., Kaletha, M. S., & Todaria, N. P. (1997). Allelopathic exclusion of understory by some agro forestry tree crops of Garhwal Himalaya. Journal of Allelopathy, 4, 321-328.
Bhatt, P., & Chauhan, D.S. (2000). Allelopathic effects of Quercus spp. On crops of Garhwal Himalaya. Journal of Allelopathy, 4, 312-318.
Dabgar, Y. D., & Kumbhar, B. A. (2010). Effects of aqueous extracts of Euphorbia thiamifolia on germination and seedling growth of Cajanus cajan. Journal of Bioscience Research, 1(3), 212-215.
Einhellig, F. A. (1995). Allelopathy-current status and future goals: In Allelopathy: Organisms, Processes, and Applications. Washington, DC: American Chemical Society Press), 1–24.
Fragasso, M., Iannucci, A., & Papa, R. (2013). Durum wheat and allelopathy: toward wheat breeding for natural weed management. Frontiers in Plant Science, 4, 375.
Jabran, K., Mahajan, G., Sardana, V., & Chauhan, B.S. (2015). Allelopathy for weed control in agricultural systems. Crop Protection, 72, 57–65.
Jadhar, B. B., & Gayanar, D. G. (1992). Allelopathic effects of Acacia auriculiformis on germination of rice and cowpea. Indonesia Journal of Plant Physiology, 1, 86-89.
Kayode, J., & Adanlawo, I. G. (1997). Allelopathic effects of aqueous extracts of Euphorbia heterophylla on radicle and plumule growth of cowpea (Vigna unguiculata) Walp. Bioscience Research Communications, 10, 23-26.
Kleiner, K. W., Raffa, K. F., & Dickson, R. E. (1999). Partitioning of 14C-labeled photosynthate to allelochemicals and primary metabolites in source and sink leaves of aspen: evidence for secondary metabolite turnover. Oecologia, 119, (3), 408–418,
Li, Z. H., Wang, Q., Ruan, X., Pan, C. D., & Jiang, D. A. (2010). Phenolics and plant allelopathy. Molecules, 15, 8933–8952.
Macias, F. A., Marin, D., Oliveros-Bastidas, A., Varela, R. M., Simonet, A. M., & Carrera, C. (2003). Allelopathy as a new strategy for sustainable ecosystems development. Biological Science in Space, 17, 18–23.
Mahmoud, S. S., & Croteau R. B. (2002). Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends in Plant Science, 7, 366–373.
Rice, E. L. (1974). Some role of allelopathic compounds in plant communities. International Journal of Current Microbiology and Applied Science, 5, 201-206.
Saeid, A., Mohammad, S., & Rida, S. (2010). Allelopathic effects of spurge (Euphorbia hierrosolymitana) on wheat (Triticum durum). American Eurasian Journal of Agriculture and Environmental Science, 7(3), 298- 302.
Thi H. L., Hyuk P., Ji P. Y., (2015). Allelopathy in Sorghum bicolor (L.) Moench: A review on environmentally friendly solution for weed control. Research on Crops, 16, 4, 657–662.
Tomaszewski, M., & Thimann, K. V. (1966). Interaction of phenolic acids, metallic ions and chelating agents on auxin induced growth. Journal of Plant Physiology, 41,1443-1454.
Weston, L. A., & Duke, S. O. (2003). Weed and crop allelopathy. Critical Review in Plant Science, 22, 367–389.
Zeng, R. S. (2014). Allelopathy-the solution is indirect. Journal of Chemistry and Ecology, 40, 515–516.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Agriculture and Environmental Science Academy

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




