Isolation, screening and molecular identification of antagonistic bacteria against Colletotrichum gloeosporioides in mango
DOI:
https://doi.org/10.26832/24566632.2022.0703018Keywords:
Antagonistic bacteria, Isolation, Molecular identification, MangoAbstract
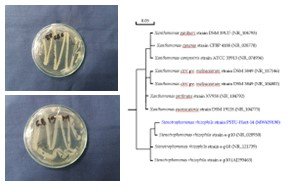
The study was conducted at the Postharvest and plant Biotechnology laboratory, Department of Horticulture, Patuakhali Science and Technology University, Patuakhali, Bangladesh during the period from January to July 2017 to isolate, screening and molecular identification of antagonistic bacteria against anthracnose of mango. All treatments were arranged in a completely randomized design (CRD) with replications and repeated twice. Epiphytic bacteria, isolated from leaf and fruit surfaces of mango, were tested as biocontrol agent against anthracnose disease caused by Colletotrichum gloeosporioides wherein 20 strains were confirmed as antagonistic. Molecular characterization of the three potential strains of bacteria were done by the amplification of 16S rDNA gene following the extraction of genomic DNA, polymerase chain reaction (PCR) amplification, gel electrophoresis and gel documentation. The PCR amplified products and the genomic DNA samples were sent to the Macrogen Company through Sunchon National University, Seoul, South Korea for molecular identification by sequence analysis. Among the 20 antagonistic bacteria screened in vitro by dual and concomitant tests, two isolates, namely GB6 (PSTU-Hort-8), and GB19 (PSTU-Hort-14) were recognized as antagonistics to the test fungus. Using the molecular identification systems, isolated bacterial strains PSTU-Hort-8 was identified as B. subtilis with National Center for Biotechnology Information (NCBI) accession numbers MW659188; on the other hand, strain PSTU-Hort-14 was identified as Stenotrophomonas rhizophila with NCBI accession number MW659190.
Downloads
References
Bally, I. S. E., Hofman, P. J., Irving, D. E., Coates, L. M., & Dann, E. K. (2009). The effects of nitrogen on postharvest disease in mango (Mangifera indica L. ‘Keitt’). Acta Horticulture 820, 365–370.
BBS, (Bangladesh Bureau of Statistics) (2016). Statistical Year Book of Bangladesh, Stat Div. Minis. Planning. Govt. People Republic, Bangladesh, Dhaka, Bangladesh. Bangladesh. pp. 202-203.
Di Francesco, A., Martini, C., & Mari, M. (2016). Biological control of postharvest diseases by microbial antagonists: how many mechanisms of action. European Journal of Plant Pathology, 145, 711–717.
Dukare, A.S., Paul, S., Nambi, V.E., Gupta, R.K., Singh, R., Sharma, K., & Vishwakarma, R.K. (2018). Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Critical Reviews in Food Science and Nutrition, 1–16.
Fokkema, N. J. (1978). Fungal antagonism in the phyllosphere. Annals of Applied Biology, 89: 115–117.
Gomez, K. A., & Gomez, A. A. (1984). Statistical Procedures of Agricultural Research, 2nd Edition. J. Wiley, New York.
Hernandez-Montiel, L. G., Zulueta-Rodriguez, R., Angulo, C., Rueda-Puente, E. O., Quiñonez-Aguilar, E. E., & Galicia, R. (2017). Marine yeasts and bacteria as biological control agents against anthracnose on mango. Journal of Phytopathology, 165, 833–840.
Islam, M.T., Olleka, A., & Ren, S. (2010). Influence of neem on susceptibility of Beauveria bassiana and investigation of their combined efficacy against sweet potato whitefly, Bemisia tabaci on eggplant. Pesticide Biochemistry and Physiology, 98, 45–49.
Kai, M., Effmert, U., Berg, G., & Piechulla, B. (2007). Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch Microbiology, 187, 351–360.
Levin, H., Hazenfratz, R., Friedman, J., Palevitch, D., & Perl, M. (2018). Partial purification and some properties of an antibacterial compound from Aloe vera. Phytotherapy Research, 2, 67-69.
Ma, R. R., Wu, X. B., & Wang, R. P. (2008). Identification and phylogenetic analysis of a bacterium isolated from the cloaca of Chinese alligator. African Journal of Biotechnology, 7(13), 2128-2133.
Madhu, K. and Pradeep, K. (2016). Anthracnose: a post-harvest disease of mango. Agrica, 4, 61–66.
Math, R. K., Islam, S. M. A., Hong, S. J., Cho, K. M., Kim, J. M., Yun, M. G., Cho, J. J., Kim, E. J., Lee, Y. H., & Yun, H. D. (2010). Metagenomic characterization of oyster shell dump reveals predominance of Firmicutes bacteria. Microbiology, 79, 509–519.
Moore, E., Krusger, A., Hauben, L., Seal, S., De Baere, R., De Wachter, K., Timmis, K., & Swings, J. (1997). 16S rRNA gene sequence analyses and inter- and intrageneric relationship of Xanthomonas species and Stenotrophomonas maltophilia. FEMS Microbiology Letter, 151, 145–153.
Obagwu, J., & Korsten, L. (2003). Integrated control of citrus green and blue molds using Bacillus subtilis in combination with sodium bicarbonate or hot water. Postharvest Biology and Technology, 28, 187-194.
Saitou, N., & Nel, M. (1987). The Neighbor-Joining Method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4:406-425.
Sambrook, J., & Russel, D. W. (2001). Molecular Cloning: A Laboratory Manual, 3th ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor Seghers D, Wittebolle L, Top EM.
Sivakumar, D., Hewarathgamagae, N. K., Wilson Wijeratnam, R. S., & Wijesundera, R. L. C. (2002). Effect of ammonium carbonate and sodium bicarbonate on anthracnose of papaya. Phytoparasitica, 30(5), 486-492.
Stackebrandt, E., & Goebel, B. M. (1994). Taxonomic note, a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. International Journal of Systematic Bacteriology, 44, 846–849.
Sutton, B.C. (1992). The genus Glomerella and its Anamorph Colletotrichum. In Colletotrichum: Biology, Pathology and Control, (ed.), J.A. Bailey, M.J. Jeger, CAB International. pp. 1-25.
Wang, Y., Yu, T., Xia, J., Yu, D., Wang, J., & Zheng, X. (2010). Biocontrol of postharvest gray mold of cherry tomatoes with the marine yeast Rhodosporidium paludigenum. Biological Control, 53, 178–182.
Yeasmin, S., Kim, C. H., Islam, S. M. A. and Lee, J. Y. (2015). Population dynamics of cellulolytic bacteria depend on the richness of cellulosic materials in the habitat. Microbiology, 84, 307–318.
Yoshida, S., Hiradate, S., Tsukamoto, T., Hatakeda, K., & Shirata, A. (2001). Antimicrobial activity of culture filtrate of Bacillus amyloliquefaciens RC-2 isolated from mulberry leaves. Phytopathology, 91, 181-187.
Zhang, X., Zhang, B. X., Zhang, Z., Shen, W. F., Yang, C. H., Yu, J. Q., & Zhao, Y. H. (2017). Survival of the biocontrol agents Brevibacillus brevis ZJY-1 and Bacillus subtilis ZJK-116 on the spikes of barley in the field. Journal of Zhejiang University Science Bulletin, 6, 770–777.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Agriculture and Environmental Science Academy

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




