Effect of water spraying time on nutritional quality of mung bean sprouts
DOI:
https://doi.org/10.26832/24566632.2025.1001020Keywords:
Mung bean, Spraying time, Sprout parameters, VarietyAbstract
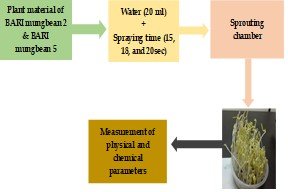
The study evaluates the effect of spraying time on growth and nutritional quality of sprouts from two mung bean varieties. The experiment was conducted using a Completely Randomized Design (CRD) with four replications. This study used two factors experiment (variety and spraying time) comprised three treatments for the two mung bean varieties, BARI Muge Bean 2 and BARI Muge Bean 5. The water spraying times were categorized T1 (15 seconds), T2 (18 seconds) and T3 (20 seconds). Data were collected in the Plant Biotechnology Lab and Post Harvest Lab, PSTU. Significant variations were observed in the result; the highest sprout shoot length (6.05 cm) and root length (1.10 cm) were recorded in T3. Additionally, the highest fresh sprout weight was with T3 measuring (25.90 g). Regarding chemical parameters, the highest values were noted as follows: pH in T3 (6.49), Total Soluble Solids (TSS) in T1 (7.22%,), vitamin C in T3 (13.20 g), anthocyanin in T3 (77.50 mg), antioxidants content in T3 (126.40 mg), phenol content in T3 (146.72 mg), carbohydrate in T3 (6.08 g), total sugar in T1 (4.22 g) and reducing sugar in T1 (2.17 g). In conclusion, the combination of longer spraying time and the BARI Mung Bean 5 variety produced higher quality sprouts and enhanced biochemical content, with the exception of pH, TSS, and sugar levels. Future research should explore additional factors affecting sprout quality.
Downloads
References
Amitrano, C., Arena, C., De Pascale, S., & De Micco, V. (2020). Light and Low Relative Humidity Increase Antioxidants Content in Mung Bean (Vigna radiata L.) Sprouts. Plants, 9. https://doi.org/10.3390/plants9091093
Chanda, S., & Dave, R. (2009). In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: An overview. African Journal of Microbiology Research, 3(13), 981-996.
Duhan, A., Khetarpaul, N., & Bishnoi, S. (1999). Optimum domestic processing and cooking methods for reducing the polyphenolic (anti nutrient) content of pigeon peas. Nutrition and health, 13(4), 227-234.
Dupré, M., Michels, T., & Le Gal, P. Y. (2020). Crop drivers in the shift from synthetic inputs to alternative practices in diversified farming systems. European Journal of Agronomy, 120, 126146.
El-Essawy, H., Nasr, P., & Sewilam, H. (2019). Aquaponics: a sustainable alternative to conventional agriculture in Egypt–a pilot scale investigation. Environmental Science and Pollution Research, 26, 15872-15883.
Fernandez-Orozco, R., Piskuła, M., Zieliński, H., Kozłowska, H., Frías, J., & Vidal-Valverde, C. (2006). Germination as a process to improve the antioxidant capacity of Lupinus angustifolius L. var. Zapaton. European Food Research and Technology, 223, 495-502. https://doi.org/10.1007/S00217-005-0229-1
Gan, R. Y., Chan, C. L., Yang, Q. Q., Li, H. B., Zhang, D., Ge, Y. Y., & Corke, H. (2019). Bioactive compounds and beneficial functions of sprouted grains. In Sprouted grains (pp. 191-246). AACC International Press.
Gomez, K. A., & Gomez, A. A. (1984). Statistical procedures for agricultural research. John wiley & sons.
Hsu, C. K., Chiang, B. H., Chen, Y. S., Yang, J. H., & Liu, C. L. (2008). Improving the antioxidant activity of buckwheat (Fagopyrum tataricm Gaertn) sprout with trace element water. Food Chemistry, 108(2), 633-641.
Khyade, V. B., & Jagtap, S. G. (2016). Sprouting exert significant influence on the antioxidant activity in selected pulses (black gram, cowpea, desi chickpea and yellow mustard). World Scientific News, (35), 73-86.
Kyraleou, M., Koundouras, S., Kallithraka, S., Theodorou, N., Proxenia, N., & Kotseridis, Y. (2016). Effect of irrigation regime on anthocyanin content and antioxidant activity of Vitis vinifera L. cv. Syrah grapes under semiarid conditions. Journal of the Science of Food and Agriculture, 96(3), 988-996.
Lane and Eynon (1990). “Method 923.09: invert sugar in sugars and syrups, lane eynon general volumetric method, final action,” in Official Methods of Analysis of Association of Official Analytical Chemists, Better World Books, Reno, NV, USA, 15th edition.
Lim, I., Kim, B. C., Park, Y., Park, N. I., & Ha, J. (2022). Metabolic and developmental changes in germination process of mung bean (Vigna radiata (L.) r. wilczek) sprouts under different water spraying interval and duration. Journal of Food Quality, 2022(1), 6256310.
Lorenza, R. (2023). Penerapan Model Predator-Prey pada Proses Perkecambahan Biji Kacang Hijau. Indonesian Journal of Applied Mathematics, 2(2), 44-50.
Ranganna, S. (1986). Handbook of analysis and quality control for fruit and vegetable products. Tata McGraw-Hill Education.
Shi, H., Nam, P. K., & Ma, Y. (2010). Comprehensive profiling of isoflavones, phytosterols, tocopherols, minerals, crude protein, lipid, and sugar during soybean (Glycine max) germination. Journal of agricultural and food chemistry, 58(8), 4970-4976.
Sims, D. A, Gamon, J. A. (2002). Relationship between leaf pigment content and spectral reflectance across a wide range species, leaf structures and development stages. Remote Sensing and Environment, 81, 337-354.
Noda, T., Takigawa, S., Matsuura-Endo, C., Saito, K., Takata, K., Tabiki, T., & Yamauchi, H. (2004). The physicochemical properties of partially digested starch from sprouted wheat grain. Carbohydrate Polymers, 56(3), 271-277.
Tang, D., Dong, Y., Ren, H., Li, L., & He, C. (2014). A review of photochemistry, metabolite changes, and medicinal uses of the common food mung bean and its sprouts (Vigna radiata). Chemistry Central Journal, 8, 1-9.
Uppalwar, S. V., Garg, V., & Dutt, R. (2020). Seeds of mung bean (Vigna radiata (L.) R. Wilczek): taxonomy, phytochemistry, medicinal uses and pharmacology. Current Bioactive Compounds, 16(9), 1–14.
Verma, G., Kumawat, N., & Morya, J. (2017). Nutrient management in mung bean [Vigna radiata (L.) Wilczek] for higher production and productivity under semi-arid tract of Central India. International Journal of Current Microbiology and Applied Sciences, 6(7), 488-493.
Waliat, S., Arshad, M. S., Hanif, H., Ejaz, A., Khalid, W., Kauser, S., & Al-Farga, A. (2023). A review on bioactive compounds in sprouts: extraction techniques, food application and health functionality. International journal of food properties, 26(1), 647-665.
Xu, M. J., Dong, J. F., & Zhu, M. Y. (2005). Effects of germination conditions on ascorbic acid level and yield of soybean sprouts. Journal of the Science of Food and Agriculture, 85(6), 943-947.
Xue, Z., Wang, C., Zhai, L., Yu, W., Chang, H., Kou, X., & Zhou, F. (2016). Bioactive compounds and antioxidant activity of mung bean (Vigna radiata L.), soybean (Glycine max L.) and black bean (Phaseolus vulgaris L.) during the germination process. Czech Journal of Food Sciences, 34, 68-78. https:doi.org/10.1722/434/2015-CJFS
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Agriculture and Environmental Science Academy

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.




